 |
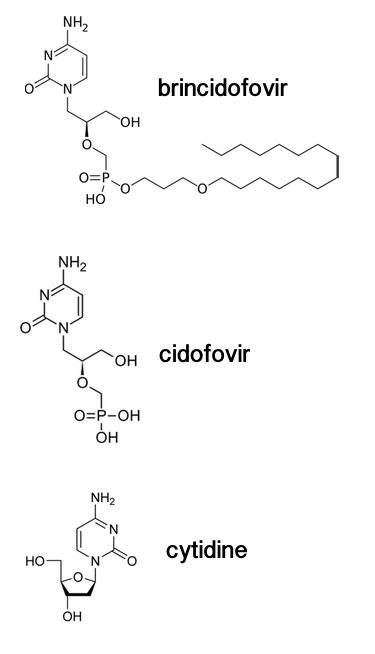
| Brincidofovir is a prodrug of cidofovir, a very hepatotoxic nucleoside analog. [Source: virology.ws] |
Adenovirus is not usually on the list of deadly viruses that Ebola and HIV-1 inhabit. However, in transplant patients who have suppressed immune systems, adenovirus infection has a 60-80% mortality rate. There are currently no FDA-approved drugs against adenovirus. The current standard of care is cidofovir, a nucleoside analog with a high risk of kidney damage. It was approved for use in cytomegalovirus retinitis in AIDS patients.
The pharmaceutical company Chimerix has developed an experimental antiviral that may offer a safer alternative. Brincidofovir is a prodrug of cidofovir. Chimerix uses a new lipid delivery technology that involves binding cidofovir with a lipid molecule. This conferees two major advantages: (1) it makes the drug absorbable by the gut (you can take it in a pill!) and (2) it can help target specific cells, where the cidofovir can be released, greatly enhancing the safety of the drug.
The question is how can Chimerix test the safety and efficacy of this new antiviral? The gold standard for clinical trials is a double blind trial in which one group receives the drug and another receives a placebo. However, when adenovirus infection has such a high mortality rate, is it ethical to give patients placebos? In response to this issue, Chimerix has received approval to conduct a late-stage clinical trial using “historic controls” instead. This type of study enables Chimerix to give brincidofovir to all patients who need it and compare their results with historic data. To keep these comparisons consistent, adenovirus patients will only be compared with other patients who received the same type of transplants and were treated at the same sites. The early data looks promising as it shows brincidofovir reduced the mortality of adenovirus infections by more than half.
Brincidofovir is also one of several experimental drugs approved for emergency use in the Ebola epidemic. Late-stage clinical trials for both adenovirus and CMV infection are moving forward, and there have been indications that it is useful in treating other viruses too, which is great news for transplant patients who often have multiple opportunistic infections.
References
1 comment:
I has suffered for Human papillomavirus HPV) for 2years, I was given some tablets at the hospital but I refused to take it, They said I have to be on it for life so I don't want take a drugs everyday for life. No point in taking medicine everyday when u won't get cure from it and I was advice to seek for natural herbal cure, after some time I found dr onokun is the most trustful herbalist that have herbs to cure wicked symptom's,I emailed dr onokun, for 2weeks been his patient he cured my (HPV) with his herbal. I only used his natural herbs for two weeks it was 100% cure. I'm not (HPV) patient anymore. I'm happy about it i finally got cured out of this mess been in my body for 2years. I also recommend you if you're living with (HPV) or herpes symptoms i also want you to be free contact dr onokun with the email attach to my post. Dronokunherbalcure@gmail.com
Post a Comment