Pocapavir is a new drug being developed to help with Polio
eradication. Currently the modified polio virus contained in the vaccine can be
shed from people with immune deficiency. Thus there are fears that the modified
polio virus will be shed from an inoculated person and enter the environment
and mutate into an infectious strain that the vaccine cannot provide immunity
for. The drug Pocapavir hopes to prevent this possibility by utilizing a protease
inhibitor to stop viral replication in the cell and a capsid inhibitor to
prevent viral capsid formation. This drug is currently under phase 2 clinical
trials for this specific application. But the even more interesting possibility
is that this drug can be a therapeutic for a virus that has not even been
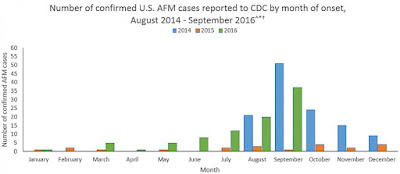
identified yet. Acute flaccid myelitis (AFM) is a disease that causes polio
like symptoms in a person but unfortunately the cause of the disease has not
been conclusively identified. Many researchers have a hunch that it is caused
by an enterovirus which is in the same family as polio, picornaviridae. Thus is
2014 the FDA allowed the usage of Pocapavir as a treatment to children in
Colorado where an outbreak of AFM occurred, under a compassionate-use basis
even while the cause of the disease was unclear. Benjamin Greenberg, a
neurologist who has worked with the drug stated that the drug had a relatively
weak but measurable impact on the viral replication. While there has been a
growth of cases of AFM in the US the FDA is no longer allowing the company to
offer the drug under the compassionate-use basis as they are now requiring the
company to apply for a New Drug Application for its usage as a AFM therapeutic.
With growing concern of AFM and one death linked to the disease occurring last
Sunday it will be interesting to see if the FDA continues to be stern with the testing
of pocapavir in a compassionate usage basis.
-Vander Harris
Links:
No comments:
Post a Comment